Highlights
Two independent, peer reviewed, technical studies were recently published that further illustrate the economic potential of Halleck Creek:
- Minerals Engineering – (Liu, et. al., 2024)
- 80% of Rare Earth Elements (“REE”s) extracted with low temperature, direct acid leaching.
- Fast leaching kinetics are attributable to Halleck Creek metamict allanite structure, with 65% of REEs extracted in the first 10 minutes of leaching.
- Halleck Creek ore is less refractory than monazite or bastnaesite, which cannot be leached using low-temperature acid tank leaching.
- Green and Smart Mining Engineering - (Xiao and Zhang, 2024)
- High-grade rare-earth enriched Halleck Creek ore highlights its economic potential.
- Density and magnetic separation techniques have demonstrated effectiveness in separation.
DENVER, May 06, 2024 (GLOBE NEWSWIRE) -- American Rare Earths (ASX: ARR | OTCQX: ARRNF and AMRRY) (“ARR” or the “Company”) is pleased to announce results from a metallurgical study on leaching extraction of REE from Halleck Creek ore by low temperature, direct acid leaching. The U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy (“EERE”) awarded a three-year (2022-2025) research contract to Phinix, LLC with American Rare Earth and Virginia Tech as team members. This metallurgical study was carried out by researchers at the Department of Mining and Minerals Engineering at Virginia Tech. These positive results further confirm the findings of the metallurgical testwork carried out by Wood PLC1 which were previously announced by ARR.
Donald Swartz, Chief Executive Officer of American Rare Earths commented:
“We’re extremely pleased with the leaching results which further validate the recent scoping study assumptions and our optimism around the project. High REE recovery at low temperatures with short duration indicate low(er) operating costs and effective extraction of rare earths. ARR is pleased that we have been able to work with the Department of Energy, Phinix and Virginia Tech on this project and look forward to continuing the relationship in the next phases of the EERE project.”
Dr. Wencai Zhang, of Virginia Tech, commented on the results:
“The low-crystallinity characteristic of the allanite can significantly reduce the extraction costs of REEs from the deposit. I am very excited about our recent findings.”
Technical Summary
Under the direction of Wencai Zhang, Ph.D., Dr. Wei Liu and doctoral candidate Zhongqing Xiao performed leaching testwork and published the results (Liu, et. al., 2024) at Virginia Tech for the project. Virgnia Tech used finely ground sample material from the Halleck Creek Project, p80 = 47.9 µm, that was concentrated using magnetic separation to produce a mixed rare earth concentrate (“Concentrate”). The leaching testwork compared the effects of a variety of acids, acid concentrations, temperatures, solid/liquid ratios, and particle sizes, collectively known as “leach kinetics”, on the recoverability of REE from the Concentrate. Virginia Tech demonstrated that approximately 80% of REE were extracted from the Concentrate using 1 M sulfuric acid at 75° C for 2 hours. Importantly, this work shows that the REE can be successfully recovered using mildly acidic, readily available chemicals at low temperatures which might decrease capital and operating costs and potentially reduce environmental impacts of the project. The study also demonstrated that the amorphous crystalline structure of the Halleck Creek allanite due to the metamictization of the allanite over geologic time further enhances the leachability of the ore.
This phase of testwork focused on the leaching kinetics of the Concentrate. During this stage of the EERE project, Virgnia Tech did not attempt to isolate discrete rare earth elements. As the project continues, Virginia Tech will focus on isolating discrete REE.
Allanite is the primary REE bearing mineral at the Halleck Creek project. Importantly, allanite is less refractory than monazite or bastnaesite, as monazite and bastnaesite cannot be leached using low-temperature acid tank leaching. No significant silica gel formation was observed during leaching. Allanite from Halleck Creek exhibits “metamict”, amorphous texture, resulting from decomposition of the crystalline structure of the mineral due to low-level decay of uranium and thorium over a 1.4-billion-year period. The amorphous nature of the Allanite appears to enhance REE leaching. Virginia Tech performed leach tests on allanite concentrates at elevated temperatures. Figure 4, below, illustrates that REE recovery decreases with increased temperature particularly when exceeding 900° C. Virginia Tech hypothesizes that allanite recrystalizes at higher temperatures, and reduces the ability to leach REE from the Concentrate. A novel, comprehensive allanite review paper prepared by leading researchers on allanite leaching supports this hypothesis and highlights the potential of allanite as an REE ore mineral (Xiao and Zhang, 2024). The allanite review paper also suggests that future metallurgical research focusing on separation and leaching is needed (Xiao and Xhang, 2024). We would encourage readers to review this paper, which is the most comprehensive review and summary of allanite to date.
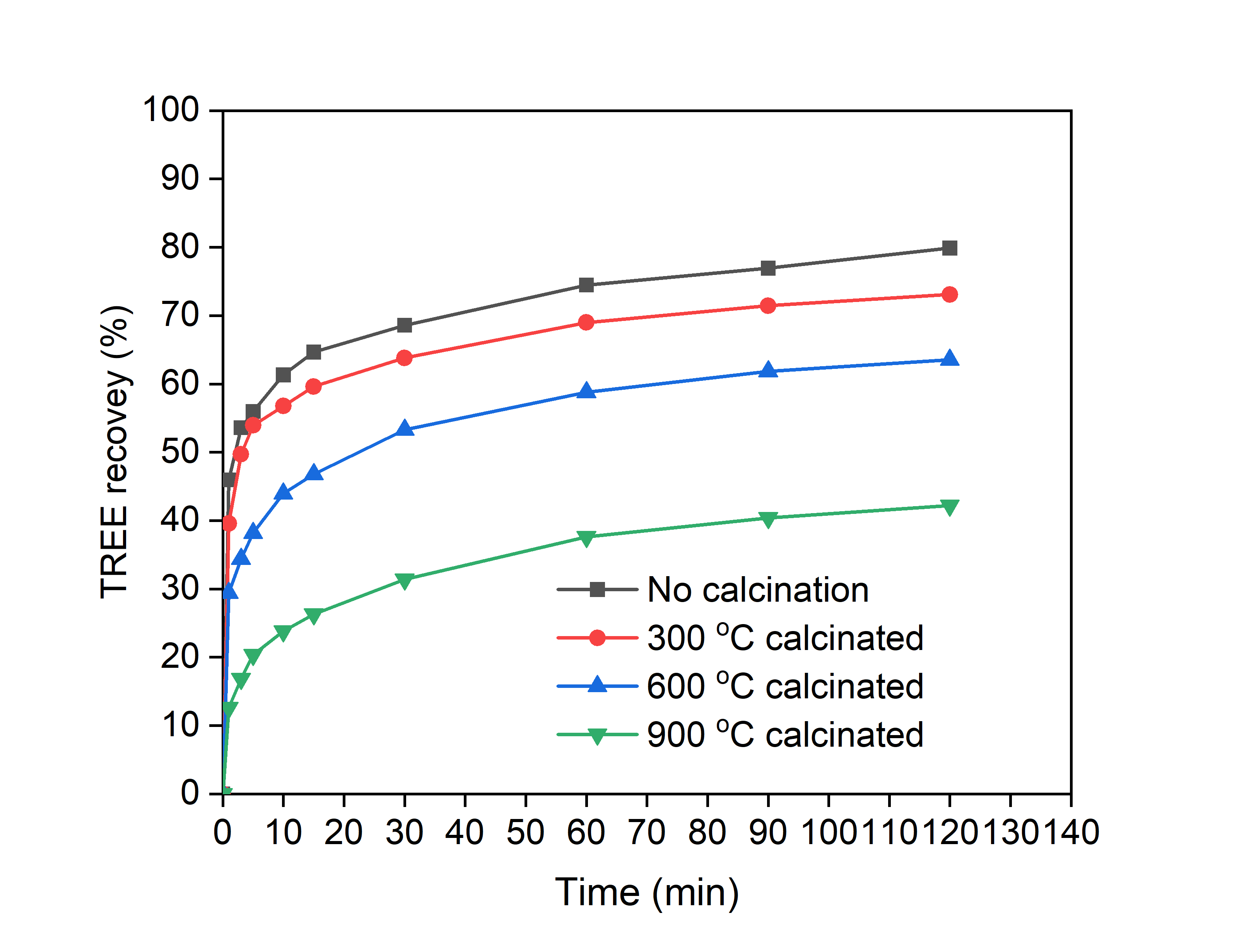
Figure 1. The effect of roasting on the recovery of REE from the allanite feed sample. The allanite feed sample was roasted in a muffle furnace for two hours. Leaching conditions: 1 M H2SO4, d80 = 47.9 µm, 75 ˚C, S/L ratio = 25 g/L, and 2 h.
References
Liu, W., Xiao, Z., Das, S., & Zhang, W. (2024). Mechanism and kinetic study of rare earth extraction from allanite by direct acid leaching. Minerals Engineering, 205, 108489.
Xiao, Z. & Zhang, W. (2024). Review of allanite: Properties, occurrence and mineral processing technologies. Green and Smart Mining Engineering, 1, 40-52.
This market announcement has been authorized for release to the market by the CEO of American Rare Earths.
See here for additional technical details and the full technical studies.
Competent Persons Statement:
This work was reviewed and approved for release by Mr. Kelton Smith (Society of Mining Engineers #4227309RM) who is employed by Tetra Tech and has sufficient experience which is relevant to the metallurgical testing and type of deposit under consideration and to the activity which he is undertaking to qualify as a Competent Person as defined in the 2012 JORC Code. Mr. Smith consents to the inclusion in the report of the matters based upon the information in the form and context in which it appears.
About American Rare Earths Limited:
American Rare Earths (ASX: ARR | OTCQX: ARRNF and AMRRY) owns the Halleck Creek, WY and La Paz, AZ rare earth deposits which have the potential to become the largest and most sustainable rare earth projects in North America. American Rare Earths is developing environmentally friendly and cost-effective extraction and processing methods to meet the rapidly increasing demand for resources essential to the clean energy transition and US national security. The Company continues to evaluate other exploration opportunities and is collaborating with US Government-supported R&D to develop efficient processing and separation techniques of rare earth elements to help ensure a renewable future.
Further information
Head Office
American Rare Earths Ltd
1658 Cole Blvd, Suite G30
Lakewood, CO, 80401
info@americanree.com
www.americanree.com
Susan Assadi
Media Relations US
sassadi@americanree.com
347 977 7125
Beverly Jedynak
Investor Relations US
Beverly.jedynak@viriathus.com
312 943 1123
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/76e39d49-56ea-4bb8-aa02-3edc795565fc







