HARRISON TOWNSHIP, MI / ACCESSWIRE / May 8, 2024 / NRx Pharmaceuticals, Inc. (NASDAQ:NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has the potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.

NRXP has achieved a series of important milestones over the past several months in the company's progression towards the final approval and commercial marketing of its products in development. NRXP has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Key issues NRXP has discussed in its recent news flow include the following topics:
Filing for FDA Approval of Intravenous Ketamine
Based on obtaining data from 4 randomized controlled trials of intravenous ketamin, NRx initiated manufacture of ketamine in order to seek FDA approval. Investors frequently fail to recognize that much of the drug approval process focuses on formulation, manufacture, sterility, and packaging of that drug, particularly in the case of sterile, injectable drug products. Currently there is only one US manufacturer of IV ketamine and the FDA has reported that ketamine faces drug shortages in the US. NRx has now formulated ketamine in partnership with Nephron Pharmaceuticals of West Columbia, SC and will complete the required three manufacturing lots this quarter. At that point, NRx is legally able to file for New Drug Approval of its IV ketamine product.
Development of New, Proprietary Formulation of HTX-100 (IV Ketamine)
Although ketamine is widely used in an intravenous formulation, IV infusions require specialized personnel and equipment not found in most doctor's offices. Subcutaneous use of ketamine is not feasible because the currently-approved formulation is highly acidic and attempts to neutralize the current formulation lead to precipitation of ketamine from solution. Acidic substances are tolerated when diluted for intravenous use, but cause pain and may cause skin ulcers if administered subcutaneously. On April 15th NRXP announced that the Company has developed a novel, proprietary formulation of IV Ketamine for use as HTX-100. This new, patentable NRXP formulation has the key advantage of achieving neutral pH, in contrast to the acidic pH of generic formulations of ketamine. NeuroRx, Inc. previously executed a joint development agreement with a manufacturer of insulin pumps but has been awaiting a suitable, pH neutral formulation of ketamine.
With this proprietary formulation, developed with partner Nephron Pharmaceuticals, a leading sterile products manufacturer, NRXP is expected to generate one or more patents, such as composition of matter or formulation. HTX-100 is expected to be marketed by HOPE Therapeutics, Inc., a wholly owned subsidiary of NRx.
Successful data readout of NRX-101 in Suicidal Treatment Resistant Bipolar Depression
On May 5th NRXP announced successful results of its clinical trial of NRX-101 vs. lurasidone in the treatment of suicidal bipolar depression. The study demonstrated that both drugs were potent antidepressants, achieving 50% rates of remission in patients with the most severe levels of depression as measured by the Montgomery Åsberg Depression Rating Scale (MADRS). However, lurasidone caused a significant rate of a life-threatening side effect called Akathisia, which is closely linked to suicide. NRX-101, on the other hand decreased akathisia scores from their baseline values to near zero levels that would be expected with placebo.
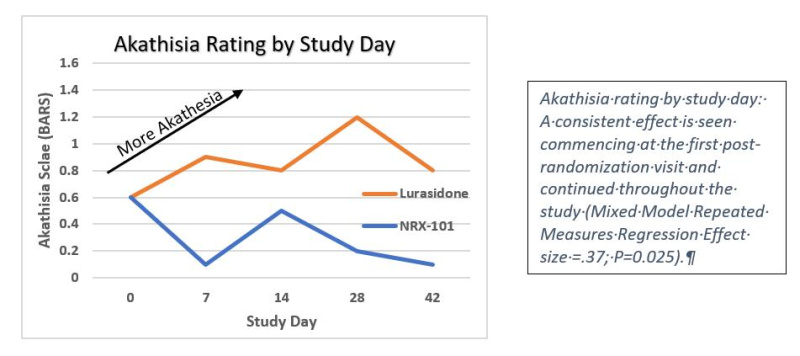
With positive data from this study and FDA comment, NRXP becomes eligible to receive the balance of its first milestone (an additional $4 million) from partners Alvogen, Inc. and Lotus Pharmaceuticals, Inc. (1745.TW). These partners would then be responsible for all future development costs in this indication. NRXP retains rights for all other indications, including chronic pain and PTSD. NRXP is then poised to receive $320 million in further milestones along with mid-teen royalties on Net Sales.
Treatment of Chronic Pain
D-cycloserine, the active ingredient in NRX-101, has been seen in a phase 2 trial to reduce chronic pain to levels previously associated with oral opioid drugs without concerns about addiction and other side effects of opioids. NRx has licensed the exclusive rights to a method patent for treatment of chronic pain with DCS from Prof. Vania Apkarian of Northwestern University.
Northwestern was granted $5 million by the US Department of Defense to conduct a randomized prospective study of DCS vs. placebo in patients with chronic low back pain. In March 2024, NRx was advised that the trial had reached data lock. Last week NRx was advised that the Institutional Review Board (IRB) of Northwestern has approved the Statistical Analysis Plan for the trial and has allowed the unblinded data to be analyzed. Top line data are expected in the next few weeks.
Treatment of Complicated UTI and Pyelonephritis with FDA Qualified Infectious Disease Product and Fast Track Designation
DCS was originally developed as an antibiotic but fell out of favor because of the potential to cause hallucinations in some patients. NRX-101 was developed, in part, because the lurasidone component blocks that undesirable side effect. Each year in the United States, 15 million people develop urinary tract infections, 20% of whom are not cured by common antibiotics and must be treated with advanced antibiotics. These patients are classified as having Complicated UTI and may develop pyelonephritis. NRx demonstrated to FDA that NRX-101 is effective in vitro against 3 resistant pathogens on the Congressionally-mandated Qualified Infectious Disease Product (QIDP) list and was granted QIDP status together with Fast Track Designation by FDA.
A critical problem with many advanced antibiotics is their propensity to cause C. Difficile infection, which leads to prolonged hospitalization and may be fatal in 10% of those over 65 who contract C. Dif. In April 2024, NRx demonstrated that NRX-101 does not disrupt the microbiome of the gut and, therefore, is unlikely to cause C Diff. Indeed, despite use in millions of patients with tuberculosis, C Diff has never been reported in association with D-cycloserine. That's because DCS is absorbed entirely in the upper GI tract and completely eliminated in the urine, rather than traveling to the gut (large intestine) like many other antibiotics.
Establishment of HOPE Therapeutics
NRXP established HOPE Therapeutics to develop and launch IV Ketamine together with related technologies with FDA New Drug Application to be submitted this year. In advance of FDA approval, HOPE is partnered with national 503b and 503a pharmacies to address the ketamine shortage declared by FDA. HOPE is planned to be spun out as a separate company to be owned by NRx, current NRx shareholders via a tax-free dividend, and new investors; Term Sheets received from prospective anchor investors for $60 million of new investment, once publicly listed
HOPE is presenting data from four randomized, prospective trials demonstrating safety and efficacy in 800 patients of IV Ketamine in treating severe and suicidal depression as the clinical basis for New Drug Application (NDA) for HTX-100 (IV Ketamine); expecting stability and CMC data sufficient for NDA filing by June 2024.
Fourth Quarter and Full Year 2023 Financial Results Plus Business Update
On April 1, 2024 NRXP announced its fourth quarter results and provided a recap of recent key business developments. These included four potential near-term milestones, including data from two clinical trials, an NDA filing and an upcoming share dividend. Additional accomplishments covered in the announcement were as follows:
NRXP delivered a 50% reduction in corporate overhead and 25% reduction in overall net loss in 2023, compared to 2024 with $0.20 per share improvement in negative earnings. Additions to working capital of $8 million in Q1 2024.
NRXP forecasts first commercial revenue in 2024 from sales of ketamine and related technologies. Company received advance of first milestone payments in 2024 for ongoing development of NRX-101 from Alvogen and Lotus Pharmaceuticals, Inc. (1975.TW)
NRXP has added over $8 million in working capital, including an advance of a $5.1 million milestone payment from partners Alvogen, Inc. and Lotus Pharmaceuticals
NRXP has elected nationally recognized attorney in highly regulated industries, and healthcare specialist, Janet Rehnquist, Esq., to the Company's Board of Directors
NRXP Management has taken actions to address NASDAQ listing compliance and naked shorting of NRx securities.
Plan to Distribute Shares of HOPE Therapeutics and Royalty Rights on Ketamine Sales to Existing NRx Shareholders
On March 18th NRXP announced that its Board of Directors has authorized its Chairman and management to take all necessary steps to affect a Dividend of HOPE Therapeutics ("HOPE") stock along with a royalty right of 1% of Ketamine sales to NRXP Shareholders and applicable warrant holders. The intent of NRXP is to distribute 49% of HOPE stock in this dividend. Shares of HOPE are planned to be publicly listed. NRx is currently completing the required audits of HOPE in preparation for filing the SEC Form 10 required for distribution of the stock dividend.
For more information on NRXP visit: https://www.nrxpharma.com/
Company Contact:
Company Name: NRx Pharmaceuticals, Inc.
Contact Person: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Country: United States
Website: https://www.nrxpharma.com/
Website: www.corporateads.com
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the on the CorporateAds website
Contact:
CorporateAds Team
Corporateads@outlook.com
SOURCE: NRXP
View the original press release on accesswire.com





